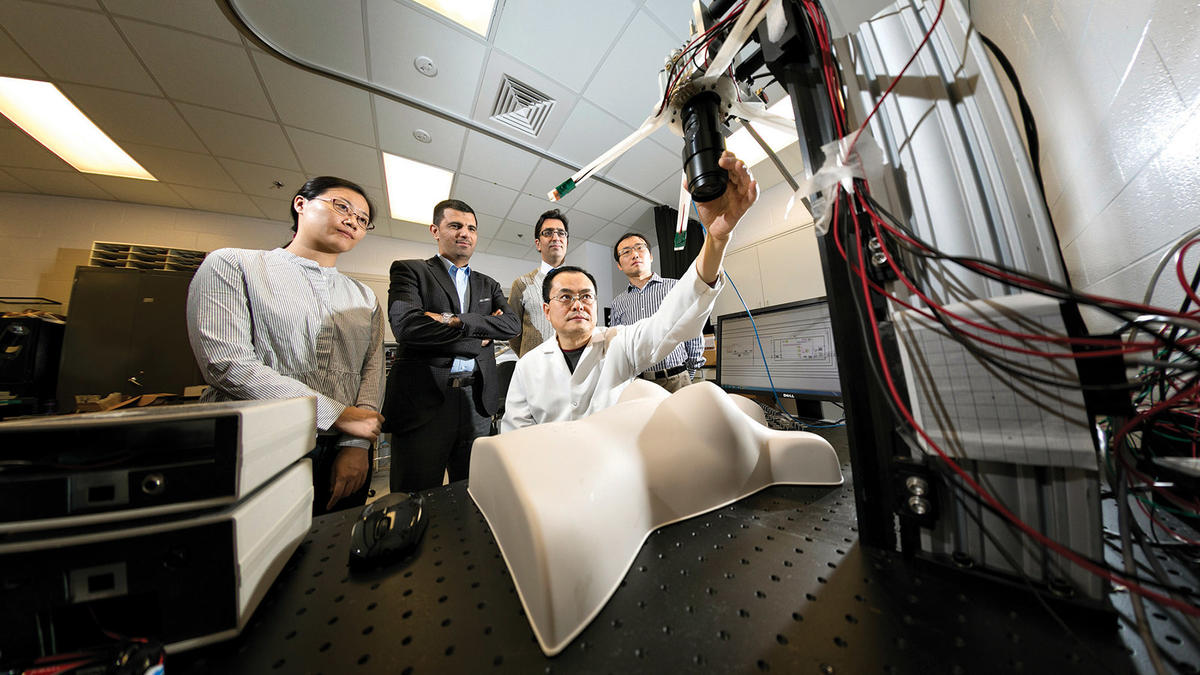
Guoqiang Yu, professor in the F. Joseph Halcomb III, M.D. Department of Biomedical Engineering, is leading a team of interdisciplinary researchers from engineering and medicine to develop, optimize and validate a noninvasive, noncontact, portable multi-wavelength speckle contrast diffuse correlation tomography (MW-scDCT) device to enable simultaneous imaging of blood flow, blood oxygenation and metabolic rate of tissue oxygen consumption in infants’ brains in the neonatal intensive care unit (NICU) and adult breasts during mastectomy in the surgical room. In 2020, his team has been awarded four grants from the National Institutes of Health that total nearly $5.7 million.
“These prestigious awards from the NIH not only reflect the high quality of the research works that Dr. Yu and his interdisciplinary teams are pursuing but also exemplify the successes in deepening engineering-medicine integration at UK-- one of the department’s missions,” said Guigen Zhang, chair of the department.
“Preterm infants are at high risk for brain injuries due to alterations in brain blood flow and oxygenation, especially after the occurrence of intraventricular hemorrhage. Meanwhile, clinical prognoses after surgical breast reconstruction often involve mastectomy skin flap necrosis or other complications associated with a lack of blood flow and oxygenation to wound tissue volumes. However, there is no reliable bedside imaging tool to monitor these functional parameters continuously in the clinic,” Yu explains. “Because we can use our MW-scDCT device at the bedside to identify ischemic and hypoxic tissues in brains and breasts more easily, continually and less expensively than with MRI or CT, we can give clinicians timely feedback on whether or not the treatments are effective.”
Noninvasive near-infrared spectroscopy and tomography technologies have been used for decades as bedside tools for continuous monitoring of tissue hemodynamics. However, most systems lack the combination of tempo-spatial resolution and wide field-of-view for real-time imaging. A few high-density tomographic systems use numerous discrete sources and detectors coupled with fiber bundles to a head cap. However, adjusting and maintaining a stable optical coupling of numerous fibers to a small fragile neonatal head for a contact measurement is labor-intensive and poses great challenges to safe head cap design. Moreover, contact measurements on wound tissues can cause infections and are not allowed in the clinic.
Yu’s solution, a patented technology (US Patent #9861319), is a noncontact probe device (scDCT) that enables fast, high-resolution imaging of blood flow distributions without ever touching the tissue.
“Applying a focused point illumination enables deep tissue penetration and using a CCD camera with thousands of pixels achieves a high-density sampling rapidly,” says Yu. “We have developed a prototype device and tested it for noncontact imaging of blood flow distributions in brains of mice, rats, piglets, and human preterm babies with different head scales, as well as human mastectomy skin flaps during surgery. Their preliminary results have verified the safety and capability of the scDCT for identifying ischemic tissue volumes and appeared in high-impact journals including NeuroImage, IEEE Transactions on Medical Imaging, Plastic and Reconstructive Surgery, Medical Physics Letters, and Journal of Biomedical Optics.”
The need for such a noncontact imaging device in the clinic, which has the potential to substantially improve healthcare, is immense. Yu is eager to meet the need. Because of the technology’s unique capability, Yu is extending the scDCT (imaging blood flow alone) to a multi-wavelength system (MW-scDCT) for simultaneous imaging of tissue blood flow, blood oxygenation and oxidative metabolism.
“Many clinical situations such as stroke expose the brain to insufficient cerebral blood flow that cannot maintain metabolic homeostasis, leading to cerebral ischemic and hypoxic stresses, and neurological disorders,” Yu recounts. “Also, effective therapeutic interventions are dependent on the findings of blood flow and metabolic improvements and eventually neural recovery. Therefore, there is an urgent need to develop robust multimodality technologies that measure all these functional parameters.”
“From detection of neonatal brain injuries or ischemic and hypoxic mastectomy skin flaps to frequent, inexpensive monitoring of treatment effectiveness, a noncontact imaging system can significantly advance the way we diagnose and treat them,” Yu concludes. “In addition, this innovative noncontact device can be used for imaging of burned, ulcerous and reconstructive tissues that cannot be measured by a contact probe.”
For more on Yu’s research, go here.